
How did life start? There may not be a bigger question. To learn the secret of our origins means going back beyond the earliest forms of biological life, past simple bacteria, and down to the chemistry of the building blocks that came earlier.
Most people have heard DNA’s double helix described as the blueprint for life, but its single-stranded relative RNA is also critical for transmitting genetic information. Both are present in the cells of all living organisms, and many scientists suspect that RNA was the original genetic material, coming on the scene before DNA, more than four billion years ago during a period scientists call “RNA world.”
But to build the RNA world, RNA and other biomolecules had to come together in the first place. Their constituent parts have a distinctive chemical property called chirality that’s related to how their atoms are arranged. And a debate has broken out about how life’s chirality got started: is it the product of the chemical environment of the early Earth, or did life inherit its chirality from space?
For some scientists, homing in on how a chain of genetic material was able to come together to start terrestrial life now involves looking away from Earth. One idea being explored in astrobiology is whether some prebiotic organic molecules could have been delivered to Earth by meteorites or dust grains. Recent discoveries in interstellar space may be providing some support for this.
In 2011, NASA published a study of meteorites suggesting that they contain nucleobases, chemicals that are components of both DNA and RNA. Thus, a critical starting material for life may have been seeded to early Earth from space. A year later, a team at the University of Copenhagen reported finding a sugar molecule in interstellar space that can be chemically transformed into ribose—the “R” in RNA. Last year, the same team uncovered a more complex molecule (methyl isocyanate) in a star-forming region more than 400 light years away from Earth.
And in 2016, two postdoctoral researchers, Brett McGuire (National Radio Astronomy Observatory, Virginia) and Brandon Carroll (California Institute of Technology), working with astronomers at the Parkes Observatory in Australia, reported the detection of a molecule in interstellar space, near the center of the Milky Way, that could have distinct consequences for the narrative of terrestrial life.

Where no chiral molecule has gone before
McGuire and Carroll discovered a molecule called propylene oxide (molecular formula: C3H6O) 25,000 light years away from Earth, in a star-forming region of our galaxy called Sagittarius B. But it wasn’t the chemical itself that was surprising; this propylene oxide bears a property that has been associated exclusively with life on Earth.
Propylene oxide is what is known as a “chiral” molecule (pronounced KY-ral, from the Greek word cheir for hand), which means that it comes in two forms: right- and left-handed. Chiral molecules have the same chemical formula, and their structures are nearly identical except for certain atoms that are attached on different sides of the three-dimensional molecule. In the case of propylene oxide, it’s the methyl group (CH3) that can attach to one of two carbons, as shown below.
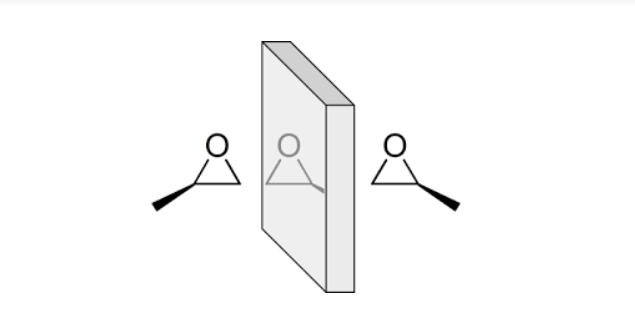
What he didn’t realize was that he happened upon a fundamental feature of organic matter: as molecules get more complex, chirality is all but guaranteed. While it doesn’t change the number or types of atoms in that molecule, the differences in how those atoms attach can impact a molecule’s function. One example is limonene, a key component of the scent of citrus fruit. The right-handed version tastes like lemon, while the left-handed one like orange. Ditto for the molecule carvone: in caraway seeds, the left-handed version binds to a receptor in neurons that line the base of your nose that send a signal to your brain telling it that it has smelled rye bread; the right-sided carvone signals your brain that it has smelled spearmint.
Beyond smell and taste, chirality determines the shape of our large-scale biological structures. The famous double helix of a DNA strand twists right, along with the sugars that comprise its backbone; the amino acids in proteins twist left. Despite the fact that these molecules naturally occur in both orientations, all the living organisms on Earth appear to have DNA that is built on the blueprint of it twisting right—perhaps descended from a single right-handed twist in the ancient RNA world.
The enzymes that help our body use amino acids and DNA bases work because they recognize the specific shapes of these molecules. An amino acid with a different chirality would have a different shape, keeping those enzymes from interacting properly with it. If you were served a burger of protein that had right-handed amino acids, your body would not be able to break it down.
This deep bias that permeates all life must have had a beginning. And McGuire and Carroll suggest that their discovery of chiral propylene oxide—as well as the earlier discoveries of methyl isocyanate and glycoaldehyde—shows that space may have had a “hand” in life’s origins.
“This is the first chiral molecule detected in outer space,” said McGuire, who is the Jansky Postdoctoral Fellow with the National Radio Astronomy Observatory. Its detection suggests that a bias toward one form of chirality is not limited to life on Earth, as has been previously thought, and lends evidence to the idea that material from elsewhere in the Solar System—possibly including some much older than Earth or even our Solar System—may have seeded the earliest chemicals necessary to form life on our planet.
Of course, chirality isn’t the only problem you have to solve—the chiral molecules we’ve seen in space are much less complex than most biomolecules.
reader comments
199