Epigenetics is one of the hottest fields in the life sciences. It’s a phenomenon with wide-ranging, powerful effects on many aspects of biology, and enormous potential in human medicine. As such, its ability to fill in some of the gaps in our scientific knowledge is mentioned everywhere from academic journals to the mainstream media to some of the less scientifically rigorous corners of the Internet.
- Wondering why identical twins aren’t actually, well, identical? Epigenetics!
- Want to blame your parents for something that doesn’t seem to be genetic? Epigenetics!
- Got a weird result from an experiment that doesn’t seem to make sense? Epigenetics!
- Want to think yourself healthy? That’s not epigenetics! (Sorry ‘bout that).
But what exactly is epigenetics – and does the reality live up to the hype?
The basics
This article includes content provided by Google. We ask for your permission before anything is loaded, as they may be using cookies and other technologies. To view this content, click 'Allow and continue'.
Epigenetics is essentially additional information layered on top of the sequence of letters (strings of molecules called A, C, G, and T) that makes up DNA.
If you consider a DNA sequence as the text of an instruction manual that explains how to make a human body, epigenetics is as if someone's taken a pack of highlighters and used different colours to mark up different parts of the text in different ways. For example, someone might use a pink highlighter to mark parts of the text that need to be read the most carefully, and a blue highlighter to mark parts that aren't as important.
There are different types of epigenetic marks, and each one tells the proteins in the cell to process those parts of the DNA in certain ways. For example, DNA can be tagged with tiny molecules called methyl groups that stick to some of its C letters. Other tags can be added to proteins called histones that are closely associated with DNA. There are proteins that specifically seek out and bind to these methylated areas, and shut it down so that the genes in that region are inactivated in that cell. So methylation is like a blue highlighter telling the cell "you don't need to know about this section right now."
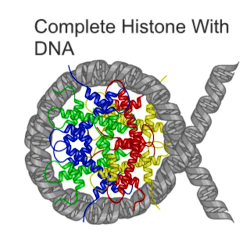
Methyl groups and other small molecular tags can attach to different locations on the histone proteins, each one having a different effect. Some tags in some locations loosen the attachment between the DNA and the histone, making the DNA more accessible to the proteins that are responsible for activating the genes in that region; this is like a pink highlighter telling the cell "hey, this part's important". Other tags in other locations do the opposite, or attract other proteins with other specific functions. There are epigenetic marks that cluster around the start points of genes; there are marks that cover long stretches of DNA, and others that affect much shorter regions; there are even epigenetic modifications of RNA, a whole new field that I’m simultaneously fascinated by and trying to ignore because it’s bound to create a lot of extra work for me in both the project manager and the grant writing parts of my role. There are no doubt many other marks we don’t even know about yet.
Even though every cell in your body starts off with the same DNA sequence, give or take a couple of letters here and there, the text has different patterns of highlighting in different types of cell – a liver cell doesn't need to follow the same parts of the instruction manual as a brain cell. But the really interesting thing about epigenetics is that the marks aren’t fixed in the same way the DNA sequence is: some of them can change throughout your lifetime, and in response to outside influences. Some can even be inherited, just like some highlighting still shows up when text is photocopied.
Epigenetics and our experiences
Any outside stimulus that can be detected by the body has the potential to cause epigenetic modifications. It’s not yet clear exactly which exposures affect which epigenetic marks, nor what the mechanisms and downstream effects are, but there are a number of quite well characterized examples, from chemicals to lifestyle factors to lived experiences:
- Bisphenol A (BPA) is an additive in some plastics that has been linked to cancer and other diseases and has already been removed from consumer products in some countries. BPA seems to exert its effects through a number of mechanisms, including epigenetic modification.
- The beneficial effects of exercise have been known for generations, but the mechanisms are still surprisingly hazy. However, there’s mounting evidence that changes to the pattern of epigenetic marks in muscle and fatty tissue are involved.
- Childhood abuse and other forms of early trauma also seem to affect DNA methylation patterns, which may help to explain the poor health that many victims of such abuse face throughout adulthood.
Epigenetic inheritance
This is an area where the hype has advanced faster and further than the actual science. There have been some fascinating early studies on the inheritance of epigenetic marks, but most of the strongest evidence so far comes from research done on mice. There have been hints that some of these findings also apply to human inheritance, but we’ve only just started to untangle this phenomenon.
- We’ve known for some time that certain environmental factors experienced by adult mice can be passed on to their offspring via epigenetic mechanisms. The best example is a gene called agouti, which is methylated in normal brown mice. However, mice with an unmethylated agouti gene are yellow and obese, despite being genetically essentially identical to their skinny brown relatives. Altering the pregnant mother’s diet can modify the ratio of brown to yellow offspring: folic acid results in more brown pups, while BPA results in more yellow pups.
- Research on the epigenetic inheritance of addictive behavior is less advanced, but does look quite promising. Studies in rats recently demonstrated that exposure to THC (the active compound in cannabis) during adolescence can prime future offspring to display signs of predisposition to heroin addiction.
- Studies of humans whose ancestors survived through periods of starvation in Sweden and the Netherlands suggest that the effects of famine on epigenetics and health can pass through at least three generations. Nutrient deprivation in a recent ancestor seems to prime the body for diabetes and cardiovascular problems, a response that may have evolved to mitigate the effects of any future famines in the same geographic area.
"More research is needed"
Epigenetics research continues apace in labs investigating a dazzling variety of topics. One interesting direction is the application of high-throughput sequencing technologies to the characterization of hundreds of ‘epigenomes’ (epigenetic marks across the entire genome). I manage a project that’s part of the International Human Epigenomics Consortium (IHEC), and am also a member of a couple of the consortium’s working groups, so I see for myself every day how fast this field is progressing. The goal of IHEC is to generate at least 1,000 publicly available ‘reference’ epigenomes (patterns of DNA methylation, six histone modifications, and gene activation) from various normal and diseased cell types. These references will serve as a baseline in other studies, in the same way that the original human genome project sequenced a reference genome to which scientists can now compare their own results to identify changes associated with specific diseases.
This is a field that’s guaranteed to keep generating headlines and catching the public’s interest. The apparent ability of epigenetics to fill some pretty diverse gaps in our understanding of human health and disease, and to provide scientific mechanisms for so many of our lived experiences, makes it very compelling, but we do need to be careful not to over-interpret the evidence we’ve collected so far. And we certainly need to be highly sceptical of anyone claiming that we can consciously change our epigenomes in specific ways through the power of thought.
Now that I’ve piqued your interest in this fascinating field (and maybe that of your unborn children. Epigenetics!), in my next piece I’ll explore the role of epigenetic changes in the onset of cancer and other diseases, and what this means for the development of new treatment options.
There are links to videos and other resources about epigenetics on the IHEC website. There’s also a free Massive Open Online Course (MOOC) in epigenetics offered by the University of Melbourne on the Coursera site; I can’t vouch for the course yet, but it looks good and I’ve signed up for the session that starts on April 28th 2014 so I can vet it for work-related purposes.
Cath Ennis is a Vancouver-based project manager and grant writer in the field of cancer genomics and epigenomics. Help her investigate the epigenetic effects of Twitter: @enniscath.
Comments (…)
Sign in or create your Guardian account to join the discussion